Porous hollow carbon nanospheres embedded with well-dispersed cobalt monoxide nanocrystals as effective polysulfide reservoirs for high-rate and long-cycle lithium–sulfur batteries†
Abstract
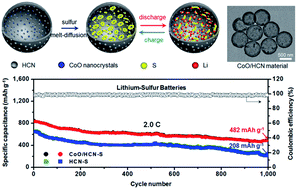
Lithium–sulfur (Li–S) batteries are promising energy storage systems owing to their high theoretical energy density and low costs due to the abundant reserves of sulfur. However, the easy dissolution of intermediate polysulfides (Li2Sx, 4 < x ≤ 8) and insulating nature of sulfur have hindered their commercialization. Herein, we develop a novel confinement approach by using porous hollow carbon nanospheres (HCNs) embedded with well-dispersed cobalt monoxide (CoO) nanocrystals (CoO/HCN), which can effectively combine the advantages of physical entrapment and chemical binding interactions of sulfur species. When used as a sulfur host material, the CoO/HCN–S composite cathode with 1.4 mg cm−2 sulfur exhibits excellent electrochemical performance with high discharge capacity (996 mA h g−1 after 200 cycles at 0.2C), great rate capability (620 mA h g−1 at 5.0C) and superior cycle stability (629 mA h g−1 after 1000 cycles at 1.0C with a capacity decay of only 0.033% per cycle and 482 mA h g−1 after 1000 cycles at 2.0C with a capacity decay of only 0.043% per cycle). Furthermore, a high and stable reversible capacity of 640 mA h g−1 after cycling for 250 cycles is achieved with a higher sulfur mass loading (3.6 mg cm−2).


 Please wait while we load your content...
Please wait while we load your content...