Thermally stable nanosized LEV zeolites synthesized by hydrothermal conversion of FAU zeolites in the presence of N,N-dimethylpiperidinium cations†
Abstract
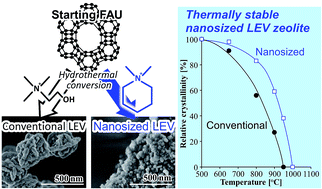
One-pot synthesis of nanosized zeolites with high crystallinity and stability is an attractive way to meet the demand for innovative catalyst and adsorbent designs. We successfully synthesized highly crystalline nanosized LEV zeolites approximately 30 nm in size that exhibited higher thermal stability than conventional LEV zeolites. The nanosized LEV zeolites were prepared by hydrothermal conversion of faujasite (FAU) zeolites in the presence of N,N-dimethylpiperidinium hydroxide as a starting silica/alumina source and an organic structure-directing agent (OSDA), respectively. It was found that the combined effect of the starting material (FAU) and a suitable OSDA yielded a suitable crystallization system of nanosized LEV zeolites with a high crystallization rate, wide synthesis window, and various aluminum contents (Si/Al = 10–30). Although the structural framework of the conventional LEV sample, with the crystal size exceeding 200 nm, nearly collapsed after thermal treatment at 950 °C for 1 h, the nanosized LEV zeolites maintained their crystallinity. The catalytic performance of protonated nanosized LEV zeolites was evaluated by studying the conversion of ethylene to propylene and butene. The nanosized LEV zeolites showed a longer lifetime owing to the suppression of catalytic deactivation arising from carbonaceous deposition on the outer surface of the crystal.


 Please wait while we load your content...
Please wait while we load your content...