Coupling of nitroxyl radical as an electrochemical charging catalyst and ionic liquid for calcium plating/stripping toward a rechargeable calcium–oxygen battery†
Abstract
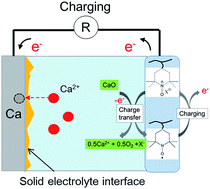
There are two problems to overcome to realize a non-aqueous rechargeable calcium–oxygen battery: lack of a catalyst for decomposing calcium oxide (CaO) as the main discharge product, and irreversible deposition/dissolution of Ca2+ at the anode. First, the catalytic capability of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO)–anion complex to decompose CaO at the O2 electrode was examined. Charge transfer from the TEMPO–anion complex to CaO occurred, leading to effective decomposition of CaO. Second, the deposition/dissolution behavior of Ca2+ was investigated in N,N-diethyl-N-methyl-N-(2-methoxyethyl) ammonium bis(trifluoromethanesulfonyl) amide at 60 °C. We observed partially reversible Ca plating/stripping under a high sweep rate in the ionic liquid. Finally we fabricated a rechargeable Ca–O2 battery using the TEMPO catalyst for the cathode, Ca chips for the anode, and an ionic liquid, and demonstrated that the battery showed repeated discharge/charge performance.


 Please wait while we load your content...
Please wait while we load your content...