Carbon-coated Li4Ti5O12 nanoparticles with high electrochemical performance as anode material in sodium-ion batteries†
Abstract
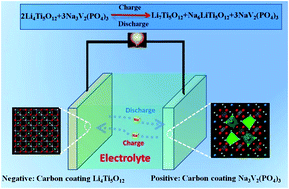
Sodium-ion batteries have been considered as promising alternatives to the current lithium-ion batteries owing to their low cost and abundant raw material. The major challenge of their practical implementation is the lack of favourable anode material. Spinel Li4Ti5O12 has been regarded as a potential anode material for its superior capability of sodium-ion storage and relatively appropriate operating voltage. However, the low intrinsic ionic and electronic conductivity of spinel Li4Ti5O12 still remains as its major drawback. Herein, carbon-coated Li4Ti5O12 nanoparticles have been synthesized through a solid-state reaction and a chemical vapour deposition method and used as an anode material for sodium-ion battery. The composite structure demonstrates excellent stability and an initial discharge specific capacity of 120.1 mA h g−1, which is maintained at 101.5 mA h g−1 after 500 cycles corresponding to 85% of capacity retention at a current density of 0.1 A g−1. In addition, a full cell was fabricated with carbon-coated Na3V2(PO4)3 as a positive electrode, which displayed discharge specific capacities of 138.5 mA h g−1 that was maintained at 114.7 mA h g−1 after 50 cycles at a current density of 0.05 A g−1, and the capacity retention was 82.8%. The results indicated that the Li4Ti5O12 nanoparticle with a carbon layer shows a promising electrochemical performance as anode materials in sodium-ion batteries.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...