Ultra-high electrocatalytic activity of VS2 nanoflowers for efficient hydrogen evolution reaction†
Abstract
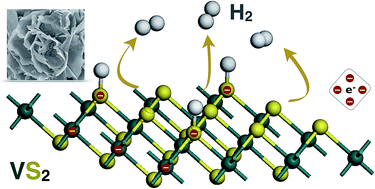
It is a great challenge to explore cheap, abundant and eco-friendly electrocatalysts for hydrogen evolution reaction (HER). Here, we report the fabrication of VS2 nanoflowers with 1T phase by a simple hydrothermal method and their electrocatalytic performance in the HER. We find that the VS2 nanoflowers show comparable HER performance to Pt in acids, including an ultra-low onset potential of 32 mV, a Tafel slope of 34 mV dec−1 which resembles that of Pt, and a small overpotential of 58 mV (54 mV for Pt) at a current density of 10 mA cm−2. High stability and almost 100% faradaic efficiency indicate the practical application of VS2 nanoflowers in the HER. Our first-principles calculations reveal that the thermoneutral Gibbs free energy of hydrogen adsorption on both the basal and edge sites of the 1T-VS2 monolayer can be achieved under certain hydrogen coverage and the monolayer shows good conductivity, which contribute to the impressive catalytic performance of VS2 nanoflowers. We expect that the VS2 nanostructures may be applicable in electrocatalysis with high efficiency.


 Please wait while we load your content...
Please wait while we load your content...