Three-dimensional printed cellular stainless steel as a high-activity catalytic electrode for oxygen evolution†
Abstract
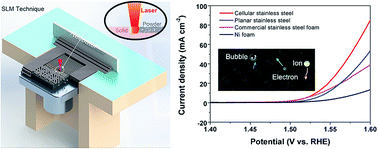
Three-dimensional (3D) porous metals are attractive electrodes for water splitting due to their high electrochemical surface areas with efficient electron/ion and bubble migration. However, the main challenges associated with the current 3D porous metals are the inability to control pore sizes, pore geometry, and pore distribution, which generally result in low electrochemical surface areas and electronic conductivity, and poor mechanical properties. Hence to effectively utilize 3D porous metals as a catalyst, a deliberate structural design is highly desirable. Herein, 3D cellular stainless steel was designed and directly printed via a selective laser melting technique. This rational 3D cellular design exhibits high mechanical strength, electronic conductivity, and electrochemical surface area, which are essential components towards efficient interconnected ion/electron/bubble transport pathways. The obtained 3D electrode exhibited excellent electrocatalytic activities for the oxygen evolution reaction with a low overpotential of ca. 302 mV to achieve a current density of 10 mA cm−2. After electrochemical activation, the overpotential decreased to 270 mV due to the formation of nickel–iron oxyhydroxides on the electrode surface. In addition to the enhanced catalytic performance, the cellular stainless steel electrodes exhibited excellent electrochemical corrosion resistance in alkaline electrolytes and other harsh electrolytes such as artificial seawater.


 Please wait while we load your content...
Please wait while we load your content...