MnCO3/Mn3O4/reduced graphene oxide ternary anode materials for lithium-ion batteries: facile green synthesis and enhanced electrochemical performance†
Abstract
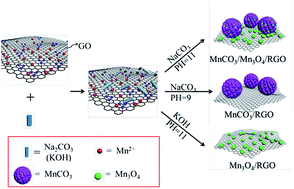
Mn-Based compounds with high reversible capacities and eco-friendliness are one of the most promising anode materials for lithium ion batteries (LIBs), but their practical applications are still hindered by low rate capability, poor cycling stability, and high cost of production. Herein, we synthesize MnCO3/Mn3O4/reduced graphene oxide (MnCO3/Mn3O4/RGO) ternary composites through a green and facile strategy, which can take full advantage of the raw materials, mitigate pollution effectively, simplify the operating procedure, and shorten the preparation time to realize large-scale preparation. When used as anode materials for LIBs, benefitting from the advantage of their structure and effective synergy among MnCO3, Mn3O4 and graphene, the ternary composites exhibit an excellent cycling stability of 988 mA h g−1 after 200 cycles at 100 mA g−1 and 532 mA h g−1 after 800 cycles at 1 A g−1, which is superior to those of binary MnCO3/RGO and Mn3O4/RGO composites. Analyses using cyclic voltammetry, charge/discharge profiles, and electrochemical impedance spectroscopy reveal improved kinetics in the electrochemical reaction of the MnCO3/Mn3O4/RGO ternary composite with cycling. Furthermore, a systematic study of the potential difference of the redox reaction provides a good explanation for the observed electrochemical performance of the ternary composites.


 Please wait while we load your content...
Please wait while we load your content...