A correlation between formation enthalpy and ionic conductivity in perovskite-structured Li3xLa0.67−xTiO3 solid lithium ion conductors
Abstract
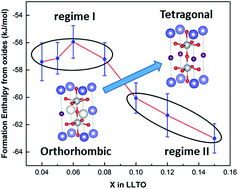
Perovskite-structured lithium lanthanum titanate (LLTO) Li3xLa0.67−xTiO3 (compositions x = 0.04 to 0.15) has been prepared by conventional solid state reaction. The phase purity and crystal structural changes were investigated with XRD, FTIR and Raman. The vibrational spectra reveal the interaction between metal cation and oxygen anion with increasing Li doping and structural evolution. LLTO and component oxides were studied by high temperature oxide melt solution calorimetry. The formation enthalpies of LLTO from oxides are exothermic for all compositions, indicating thermodynamic stability. There are two regimes in the trend of formation enthalpy with increasing Li concentration. In the first regime, x ≤0.08, the formation enthalpies vary slowly with composition, but the lowest stability by about 1.5 kJ mol−1 is seen at x = 0.06. An abrupt change in the formation enthalpy trend is observed in the second regime when x ≥0.1, where maximum lithium ion conductivity (at x = 0.10) is reported. The least stable composition, x = 0.06, occurs where maximum charge carrier concentration and lowest activation energy is reported. From the thermodynamic study, it is clear that the energetically least stable composition correlates with lowest activation energy whereas the sharp change in formation enthalpy trend correlates with highest Li-ion conductivity.


 Please wait while we load your content...
Please wait while we load your content...