Na3V2(PO4)3/C synthesized by a facile solid-phase method assisted with agarose as a high-performance cathode for sodium-ion batteries†
Abstract
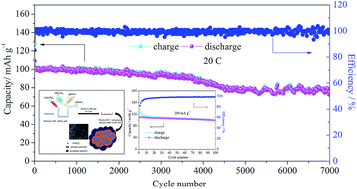
A type of Na3V2(PO4)3/C (NVP/C) cathode material for sodium ion batteries was synthesized via a facile solid-phase method. Agarose, a natural polysaccharide from seaweed that can form a 3D network structure through hydrogen bonds in polar solvents, was used as a carbon source and a medium to form NVP/C particles as nanograins with a thin layer of carbon coating in a 3D carbon network. As the cathode, the NVP/C material exhibited a reversible capacity of 116 mA h g−1 at 1C, which is very close to its theoretical capacity (117.6 mA h g−1), with a capacity retention of 97.3% after 500 cycles. At 20C, the NVP/C had a capacity of 100 mA h g−1 and still kept a relatively stable capacity of 78 mA h g−1 after being cycled 7000 times. Based on the excellent performance of the obtained cathode material, a sodium ion full cell was assembled with S-doped carbon as the anode, which delivered 110 mA h g−1 of discharge capacity at 200 mA h g−1 (calculated using NVP as the cathode) and displayed an average voltage of around 1.7 V. All of these results imply that the prepared NVP/C has potential for application in sodium ion batteries for large-scale energy storage.


 Please wait while we load your content...
Please wait while we load your content...