Phosphate tuned copper electrodeposition and promoted formic acid selectivity for carbon dioxide reduction†
Abstract
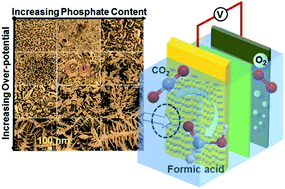
Fabrication of catalytically active electrodes by electrodeposition is attractive due to its in situ nature, easy controllability, and large-scale operation capability. Most recently, modifying the electrodes with phosphate ligands through electrodepositing electrode materials has shown promising results in improving the kinetics of some reactions. However, it is unclear how the presence of phosphate anions affects the electrodeposition process and functions in catalyzing reactions. Here, we report a systematic study on electrodeposition of Cu in the presence of phosphate anions. The phosphate anions form a complex with free Cu(II) cations, competing with the electrodeposition process. The competition between the two processes results in an insufficient supply of free Cu(II) for electrodeposition, especially at the diffusion layer. This is evidenced by the calculation of free Cu(II) concentration and the electrodeposition current at identical applied potentials. We also found that the electrodeposition of Cu in the presence of phosphate generates Cu-oxyo/hydroxyo-phosphate species on the deposited copper surface. The modified electrodes with phosphate species exhibit higher selectivity for HCOOH formation (faradaic efficiency ∼80%) from the electrochemical reduction of CO2 as compared with Cu foil (faradaic efficiency ∼33%). The effect of phosphate ligands promoting HCOOH selectivity is further verified by stripping off the ligands and regenerating the ligands.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...