Highly stable and efficient non-precious metal electrocatalysts of tantalum dioxyfluoride used for the oxygen evolution reaction†
Abstract
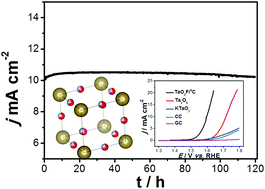
Superior stability is very important for electrocatalysts during the oxygen evolution reaction (OER) for long-term cyclic applications. Tantalum dioxyfluoride, TaO2F, as very stable compound, supported on graphitized carbon (gC) has been synthesized using a simple ion adsorbed methodology and used as an electrocatalyst for the OER in alkaline medium. The TaO2F/gC electrocatalyst exhibited efficient catalytic activity with a lower onset potential of 1.48 V vs. RHE for the OER and an overpotential of only 360 mV to achieve 10 mA cm−2. Moreover, the TaO2F/gC electrocatalyst showed superior stability and was almost unchanged after 20 000 cycles of polarization and using at a current density of 10 mA cm−2 for several days.


 Please wait while we load your content...
Please wait while we load your content...