Metal-free branched alkyl tetrathienoacene (TTAR)-based sensitizers for high-performance dye-sensitized solar cells†
Abstract
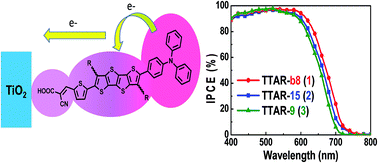
A new series of metal-free alkylated tetrathienoacene (TTAR)-based organic chromophores, TPA–TTAR–TA (R = branched-C8H17, 1, TTAR-b8; R = C15H31, 2, TTAR-15; R = C9H19, 3, TTAR-9), are synthesized for application in dye-sensitized solar cells (DSSCs). Due to the extensively conjugated TTAR π-bridge, all three dyes exhibit high extinction coefficients (1 × 105 M−1 cm−1). By systematically exploring the effects of the TTAR alkyl chain substituents, a significant influence of the dye coverage (orientation) on the TiO2 surfaces is observed. The branched-alkyl TTAR-b8 (1) promotes significant tilting and packing distortion on TiO2 in comparison to more ordered monolayers of linear long alkyls TTAR-15 (2) and TTAR-9 (3). Photophysical measurements on the dye-grafted TiO2 films reveal that the branched-alkylated TTA unit in 1 enhances the electron injection efficiency, in agreement with the high quantum efficiency. Notably, by utilizing a three-dimensional (3D) photonic crystal (PhC) layer to enhance the coherent scattering an increase the light absorption, TTAR-b8 exhibits higher short-circuit current densities and achieved a high PCE of 11.18%. TTAR-b8 is thus the best performing fused-thiophene-based organic DSSC dye reported to date.


 Please wait while we load your content...
Please wait while we load your content...