A fluorinated dialkoxide-based magnesium-ion electrolyte†
Abstract
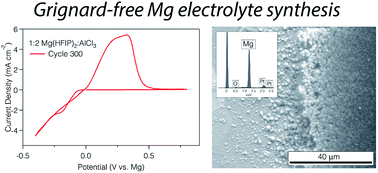
Efficient large scale electrochemical energy storage systems, such as those based on multivalent ions, are a prerequisite for the realization of intermittent renewable energy sources. From the perspectives of both cost and environmental concerns, it is of critical importance that components of these systems are synthesized using sustainable chemical processes starting from their initial conception. Herein, we report on a fluorinated dialkoxide-based magnesium-ion electrolyte that is synthesized through an atom-efficient and scalable process without the use of any metal alkyls. The electrolyte composition results in high solution conductivity (4.77 mS cm−1 at 26.3 °C), low overpotentials, ca. 100% coulombic efficiency for electrodeposition/dissolution, and good performance in full battery cells using Chevrel phase Mo6S8.


 Please wait while we load your content...
Please wait while we load your content...