A furazan-fused pyrazole N-oxide via unusual cyclization†
Abstract
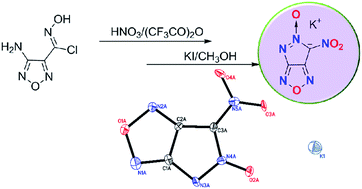
6-Nitro-pyrazolo[3,4-c]furazanate 5-oxide, a new fused anion with high energy, was designed and synthesized via an unusual intramolecular cyclization reaction of 3 by using a mixture of 100% nitric acid and trifluoroacetic anhydride followed by KI reduction. The potassium (6) and nitrogen-rich energetic (9–16) salts were prepared, and fully characterized by IR and multinuclear NMR spectroscopy, elemental analysis, and single-crystal X-ray structuring, 6, 9 and 16. Hydroxylammonium salt (10) has excellent detonation performance but high sensitivity, while 13 and 16 have detonation velocities comparable to 1,3,5-trinitro-1,3,5-triazinane (RDX), which suggests they may have potential application as green primary or secondary explosives.


 Please wait while we load your content...
Please wait while we load your content...