Reduced titania@layered double hydroxide hybrid photoanodes for enhanced photoelectrochemical water oxidation†
Abstract
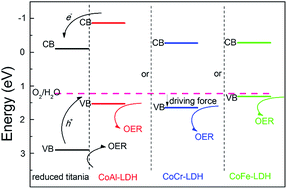
Photoelectrochemical (PEC) water oxidation has received considerable attention owing to its key role in the overall water splitting. In this work, several reduced titania@layered double hydroxide (CoAl–LDH, CoCr–LDH, and CoFe–LDH) hybrid photoanodes were fabricated via electrochemical deposition of LDH on the reduced titania, and their PEC properties for water oxidation were studied systematically. The reduced titania@CoCr–LDH photoanode shows a much improved PEC performance compared with pristine reduced titania, with a photocurrent density enhancement of 43% (from 0.65 mA cm−2 to 0.93 mA cm−2) and an onset potential decrease of 21% (from 0.23 V to 0.18 V vs. the RHE). This improvement is also successfully demonstrated in the reduced titania@CoAl–LDH and reduced titania@CoFe–LDH system. The photoconversion efficiency of reduced titania is significantly enhanced after the incorporation of LDH (0.42–0.51% at ∼0.46 V vs. the RHE). Both the experimental studies and DFT calculations confirm a synergistic effect between the reduced titania and LDH. The results show that a good match of the band structure facilitates the fast electron–hole separation and the migration of holes from reduced titania to LDH, followed by the LDH catalyzed water oxidation. The CoCr–LDH has the highest driving force for oxygen evolution among these LDHs, accounting for the optimal PEC performance of the reduced titania@CoCr–LDH photoanode.


 Please wait while we load your content...
Please wait while we load your content...