Hydrogen-induced magnesium–zirconium interfacial coupling: enabling fast hydrogen sorption at lower temperatures
Abstract
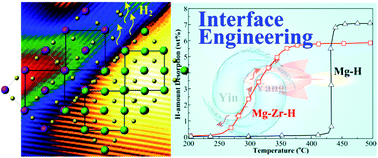
The implementation of magnesium (Mg) as a hydrogen-storage medium has long been restricted because of its rather sluggish hydrogen sorption at high temperatures. Here, we report a method for using hydrogen-induced Mg–Zr interfacial coupling to manipulate the migration of hydrogen atoms and thus tune their uptake and release in a micrometer-sized Mg-rich composite. The associated Mg–Zr–H interfaces were assembled in situ by high-pressure ball milling and isothermal treatment of MgH2 and Zr powders under a hydrogen atmosphere. The interfaces gradually disintegrated upon MgH2 desorption but also recovered their original compositions upon absorption while the ZrH2 originating from Zr hydrogenation remained completely unchanged. Compared to pure MgH2, the hydrogen sorption of the Mg–Zr–H composite was thus shown to be dramatically faster at lower temperatures, whereby it not only absorbed hydrogen close to saturation at 100 °C within 2 h, while the pure Mg did not absorb hydrogen at all, but also started to release hydrogen at ∼235 °C with a reduction in the activation energy of desorption by ∼40 kJ mol−1. These remarkable enhancements cannot be explained by the decrease in the size of the MgH2 grains alone but are most likely due to the introduction of Mg–Zr–H interfaces and large fractions of defects that provide channels for facile hydrogen dissociation and migration into the Mg/MgH2 matrix.


 Please wait while we load your content...
Please wait while we load your content...