One-pot synthesis of highly efficient MgO for the removal of Congo red in aqueous solution†
Abstract
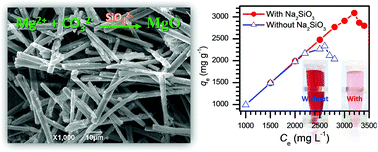
Magnesium oxide (MgO) has been demonstrated to be a promising candidate for the treatment of toxic dyes in wastewater due to its unique characteristics (e.g., high isoelectric point, nontoxicity and cost-effectiveness). However, it is still a great challenge to fabricate highly efficient MgO for toxic dyes through facile synthetic strategies. Herein, a porous rod-like MgO with extremely high adsorption capacity for Congo red dye (3236 mg g−1) has been presented through a facile precipitation reaction between Mg2+ and CO32− in the presence of a trace amount of sodium silicate. After systematically investigating the experimental parameters, the results exhibited that the performance of the resulting MgO was very sensitive to the amount of sodium silicate in the reaction, stirring time and calcination temperature, and its adsorption capacity was closely related to the surface base properties rather than the specific surface areas of MgO. The adsorption process of Congo red on the as-synthesized product obeyed the pseudo-second-order rate equation and the Langmuir adsorption model. It is expected that the developed method will provide a facile route to gram level production of highly efficient MgO for handling toxic dyes in industrial wastewater.


 Please wait while we load your content...
Please wait while we load your content...