Bimetallic Cu–Pd alloy multipods and their highly electrocatalytic performance for formic acid oxidation and oxygen reduction†
Abstract
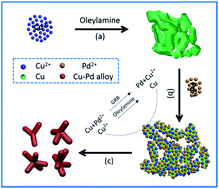
Shape-controlled synthesis of bimetallic nanomaterials is critical to the improvement of their catalytic performance for a given catalytic reaction. In this work, we demonstrate a simple and effective approach for the synthesis of bimetallic Cu–Pd alloy multipods mainly consisting of tripods, tetrapods, pentapods, and hexapods via combining the galvanic replacement reaction (GRR) between pre-synthesized Cu template nanoparticles and Pd2+ ion precursors with the reduction of the Cu2+ ions generated in the GRR by oleylamine. Benefiting simultaneously from their unique multipod structure with abundant active edges/corner atoms, electron transfer from Cu to Pd in alloys that enhances their CO tolerance, and the lattice contraction of Pd imposed by Cu, which weakens the binding strength between Pd and reaction intermediates, these Cu–Pd alloy multipods show remarkable improvement of their electrocatalytic performance not only for the oxidation of formic acid but also for the reduction of oxygen when benchmarked against spherical Cu–Pd alloy nanoparticles and commercial Pd/C catalysts. The strategy in this work may provide a method to synthesize other bimetallic alloy multipods to achieve specific functions for various applications.


 Please wait while we load your content...
Please wait while we load your content...