Tailored Li2S–P2S5 glass-ceramic electrolyte by MoS2 doping, possessing high ionic conductivity for all-solid-state lithium-sulfur batteries
Abstract
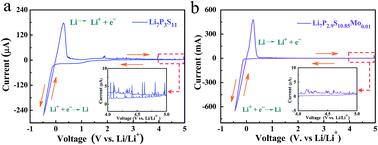
Tailored synthesis of high-quality solid electrolytes is critical for the development of advanced all-solid-state batteries. Currently, the performance of solid electrolytes is still hindered by low ionic conductivity and poor electrochemical stability. Herein, we report a novel high-quality MoS2-doped Li2S–P2S5 glass-ceramic electrolyte (Li7P2.9S10.85Mo0.01) prepared by a facile combined method of high-energy ball milling plus annealing. Impressively, the obtained Li7P2.9S10.85Mo0.01 exhibits a high ionic conductivity of 4.8 mS cm−1 at room temperature, and a stable wide electrochemical window up to 5 V (vs. Li/Li+). The MoS2-doped electrolyte is demonstrated to have more stability on the lithium metal as compared to the Li7P3S11 counterpart. In addition, all-solid-state Li-S cells are assembled based on the Li7P2.9S10.85Mo0.01 electrolyte and show a high discharge capacity of 1020 mA h g−1, better than that of a cell based on a Li7P3S11 electrolyte. Our study provides a new type of solid electrolyte for the construction of high-performance all-solid-state Li-S batteries.


 Please wait while we load your content...
Please wait while we load your content...