In situ electrochemical formation of core–shell nickel–iron disulfide and oxyhydroxide heterostructured catalysts for a stable oxygen evolution reaction and the associated mechanisms†
Abstract
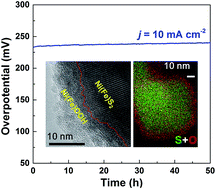
For electrochemical production of H2 fuels from water splitting, the development of efficient and economic catalysts for the oxygen evolution reaction (OER) is still a challenging issue. This is because an OER process usually involves multiple electron-transfer and reaction steps; these result in large overpotentials and significant energy loss. Thus, a smart design of highly efficient, stable and cheap OER electrocatalysts is important for the improvement of energy conversion efficiency and reduction of water splitting procedure cost. In this work, we find that a thin crystalline oxyhydroxide layer has been in situ electrochemically formed on the surfaces of conductive nickel–iron disulfide nanostructures; such a heterostructure takes advantage of highly catalytically active oxyhydroxide surfaces and excellent conductivity of the interior disulfide phase. This results in a very low overpotential of 230 mV at a current density of 10 mA cm−2, which is among the best OER catalysts in alkaline electrolyte ever reported. The crystalline oxyhydroxide layer can effectively prevent the disulfide core from further oxidation, maintains the core–shell structure of the catalyst and is considered to be critical for stable and efficient OER performances.


 Please wait while we load your content...
Please wait while we load your content...