Large CO2 uptake on a monolayer of CaO†
Abstract
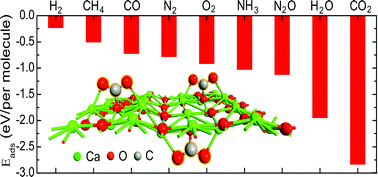
Density functional theory calculations are used to study gas adsorption properties of a recently synthesized CaO monolayer, which is found to be thermodynamically stable in its buckled form. Due to its topology and strong interaction with the CO2 molecules, this material possesses a remarkably high CO2 uptake capacity (∼0.4 g CO2 per g adsorbent). The CaO + CO2 system shows excellent thermal stability (up to 1000 K). Moreover, the material is highly selective towards CO2 against other major greenhouse gases such as CH4 and N2O. These advantages make this material a very promising candidate for CO2 capture and storage applications.


 Please wait while we load your content...
Please wait while we load your content...