3,4-Dinitro-1-(1H-tetrazol-5-yl)-1H-pyrazol-5-amine (HANTP) and its salts: primary and secondary explosives†
Abstract
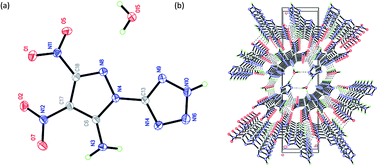
The combination of superior energetic structural fragments is a feasible route to design new energetic materials. In this work, selected metal and nitrogen-rich salts based on 3,4-dinitro-1-(1H-tetrazol-5-yl)-1H-pyrazol-5-amine (HANTP) are prepared and characterized by 1H/13C NMR, IR spectroscopy, and elemental analysis. The crystal structures of neutral HANTP (2), and its potassium (4), sodium (5), ammonium (6), and guanidinium (9) salts are determined by single-crystal X-ray diffraction, and their properties (density, thermal stability, and sensitivity towards impact and friction) are investigated. The detonation properties are evaluated by the EXPLO5 (v6.01) program using the measured density and calculated heat of formation (Gaussian 03). All compounds exhibit thermal stabilities with decomposition temperatures ranging from 171 to 270 °C, high densities (1.61–2.92 g cm−3), and high positive heats of formation (630.4–1275.2 kJ mol−1). The inorganic salts (4 and 5) assume particular structures (two-dimensional and one-dimensional metal–organic frameworks, respectively). Suitable impact and friction sensitivities and being free of toxic metals place these compounds within the green primary explosives group and several of the new organic salts exhibit detonation and other properties that compete with, or exceed the performance of those of HMX.

 Please wait while we load your content...
Please wait while we load your content...