Degradation of radiation grafted hydroxide anion exchange membrane immersed in neutral pH: removal of vinylbenzyl trimethylammonium hydroxide due to oxidation
Abstract
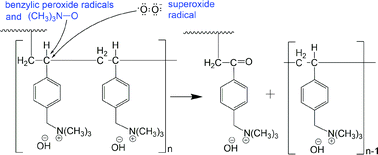
Anion exchange membranes (AEMs) fabricated using polyethylene (PE) and ethylene tetrafluoroethylene films are subjected to degradation tests in deionised water for electrolyser/fuel cell operation. After the degradation test, the decrease in ion-exchange capacity (IEC) of the AEM, hence decrease in ionic conductivity, is found to be influenced by the applied gamma radiation dose rate. The use of high radiation dose rate produces membranes with improved stability in terms of % IEC loss due to shorter, more uniformly distributed vinylbenzyl chloride (VBC) grafts. For LDPE-based AEMs, increasing the applied radiation dose rate during grafting from 30 to 2000 Gy h−1 significantly reduces AEM % IEC loss from 38 to 11%, respectively. Analyses of both the aged functionalised membranes and their resulting degradation products confirm the loss of not only the functional group, but also the VBC group, which have not been reported previously in the literature. Oxidation reactions taking place in solutions close to neutral can be the main contributor to the IEC loss in contrast to the widely reported E2 or SN2 attack on the head group in high alkalinity solutions. We therefore propose parallel degradation mechanisms to head group loss of AEM that involves peroxide radicals which is more dominant at low alkalinity solutions.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...