The organic-moiety-dominated Li+ intercalation/deintercalation mechanism of a cobalt-based metal–organic framework†
Abstract
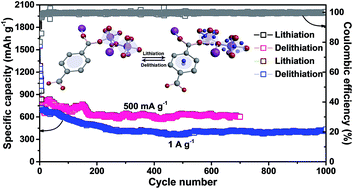
Metal–organic frameworks (MOFs) have advanced many application fields due to their intriguing structure. However, the application of MOFs in lithium-ion batteries (LIBs) is severely hindered by the lack of a detailed insight into the delithiation and lithiation behaviors of MOFs. This study employs soft X-ray spectroscopy and high-resolution solid-state NMR techniques to study the electrochemical process of a seashell-like Co-based MOF. These experiments demonstrate that Li-ions are intercalated to the carboxyl groups and benzene rings of this MOF during cycling, accompanied by the distortion of CoO6 octahedral sites. Furthermore, the Co-MOF employing this organic-moiety-dominated intercalation/deintercalation mechanism exhibits high initial coulombic efficiency (80.4%) and unprecedented long-term cyclic stability (1021 mA h g−1 at 100 mA g−1 after 200 cycles; 601 mA h g−1 at 500 mA g−1 after 700 cycles; 435 mA h g−1 at 1 A g−1 after 1000 cycles) when evaluated as the anode material in LIBs. To our knowledge, this is the longest cycle life ever reported for a MOF-based lithium ion battery anode.


 Please wait while we load your content...
Please wait while we load your content...