Hydrogen production based on a photoactivated nanowire-forest†
Abstract
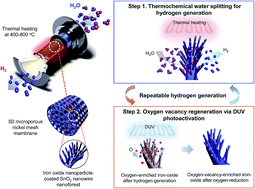
For several decades, the key challenge associated with thermochemical hydrogen generation has been the achievement of water splitting and catalyst regeneration at low temperatures while maintaining a reasonably high conversion efficiency over many cycles. Herein, we report low-temperature thermochemical hydrogen generation using hierarchically assembled iron oxide nanoarchitectures. Iron oxide nanoparticles conformally deposited onto a SnO2 nanowire forest allowed the splitting of water molecules and the production of hydrogen gas at temperatures of 400–800 °C, with a high specific gas-forming rate as high as ∼25 000 μmol per g per cycle (250 min). More remarkably, deep-ultraviolet photoactivation enabled low-temperature (200 °C) catalyst regeneration and thereby multiple cycles of hydrogen production without any significant coalescence of the oxide nanoparticles nor substantial loss of the water-splitting efficiency. Hierarchically arranged iron oxide nanoarchitectures, in combination with photochemical catalyst regeneration, are promising for practical hydrogen generation by harvesting wasted thermal energy, even at temperatures below 500 °C.

 Please wait while we load your content...
Please wait while we load your content...