Highly enhanced electrochemical performance of ultrafine CuO nanoparticles confined in ordered mesoporous carbons as anode materials for sodium-ion batteries†
Abstract
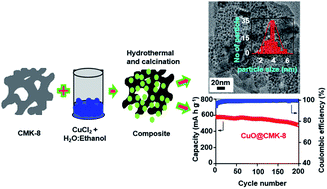
Ultrafine CuO nanoparticles are successfully encapsulated into two ordered mesoporous carbons (OMCs) with different pore architectures, namely CMK-8 with a 3D cubic mesostructure and CMK-3 with a 2D hexagonal mesostructure, and used as anodes in sodium-ion batteries. The electrochemical test results demonstrate that the CuO@CMK-8 nanocomposite with ultra-small CuO nanoparticles (around 4 nm in diameter) and a high content of CuO (78%) exhibits superior electrochemical performance as compared to that of the CuO@CMK-3 nanocomposite and the pristine CuO electrodes. The CuO@CMK-8 anode delivers an initial discharge capacity of 1405 mA h g−1 with a reversible capacity of 768 mA h g−1 at a current density of 20 mA g−1. At a current density of 100 mA g−1, it provides a reversible capacity of 477 mA h g−1 after 200 cycles with coulombic efficiency over 99%. The remarkable enhancement of the electrochemical performance of the CuO@CMK-8 nanocomposite can be attributed to the electrically conductive network of CMK-8 in the CuO@CMK-8 nanocomposite wherein the ultra-small CuO nanoparticles supported on CMK-8 act as a synergistic elastic buffer. Furthermore, cyclic voltammetry, ex situ XRD, SEM and TEM measurements provide deeper insights to the reversible conversion reaction in the CuO@CMK-8 nanocomposite system during the sodiation/desodiation process.


 Please wait while we load your content...
Please wait while we load your content...