Ultrasmall diiron phosphide nanodots anchored on graphene sheets with enhanced electrocatalytic activity for hydrogen production via high-efficiency water splitting†
Abstract
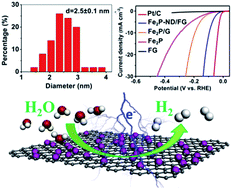
Transition metal phosphides (TMP) have been one of the excellent candidates as low-cost and high-efficiency catalysts for the sustainable hydrogen evolution reaction (HER). Nevertheless, construction of TMP with abundant exposed active sites is of urgent concern and highly desirable for the HER. Herein, we report a novel strategy to configure integrated active site-enriched architectures (Fe2P-ND/FG) composed of diiron phosphide (Fe2P) nanodots with a diameter of ∼2.5 nm uniformly anchored on porous and fluffy graphene sheets (FG). The interconnected conductive networks within porous FG favor the formation of uniformly and highly dispersed Fe2P nanodots, finally helping the promotion of fast electrolyte ion and electron transfer during the electrochemical process. Compared with bulk Fe2P, these ultrasmall Fe2P nanodots lead to abundant exposed edges and atoms. Benefiting from the highly exposed active sites derived from Fe2P nanodots and superior electrical conductivity stemming from an interconnected graphene matrix, the as-made Fe2P-ND/FG hybrids exhibit outstanding HER catalytic activity and stability. Overpotentials as low as 44 and 91 mV are required to achieve current densities of 2 and 10 mA cm−2, respectively. The present strategy provides a novel approach for configuring electrode materials with highly exposed active sites for high-efficiency energy storage/conversion devices.


 Please wait while we load your content...
Please wait while we load your content...