General synthesis of xLi2MnO3·(1 − x)LiNi1/3Co1/3Mn1/3O2 (x = 1/4, 1/3, and 1/2) hollow microspheres towards enhancing the performance of rechargeable lithium ion batteries†
Abstract
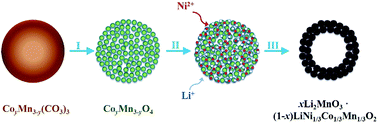
Well-crystallized and high-performance xLi2MnO3·(1 − x)LiNi1/3Co1/3Mn1/3O2 (x = 1/2, 1/3, 1/4) hollow microspheres are prepared through an in situ self-sacrificial template route. By precisely adjusting y values in the starting precursor, CoyMn3−yO4 porous microspheres, uniform xLi2MnO3·(1 − x)LiNi1/3Co1/3Mn1/3O2 hollow microspheres are formed. Property testing of lithium ion batteries shows that an appropriate x value plays an important role in their electrochemical behavior: a higher x value leads to a higher specific capacity, but a worse cycling capability, and vice versa; when x = 1/3, the xLi2MnO3·(1 − x)LiNi1/3Co1/3Mn1/3O2 electrode shows a high specific capacity and a high capacity retention rate. In particular, the hollow microspheres with x = 1/3 achieved a reversible capacity as high as 203 mA h g−1 at 0.25C and 181 mA h g−1 at 1.0C over 200 cycles. A rate capacity as high as 191 mA h g−1 at 5.0C is obtained after 5 cycles stepwise from 0.25C to 5.0C. This work provides a general approach based on the use of an in situ self-sacrificial template to synthesize xLi2MnO3·(1 − x)LiMO2 (0 < x < 1, M = Ni, Co, Mn, etc.) at various x values and other targeted materials in a morphology with hollow interiors, which is inaccessible through a wet chemistry route.

 Please wait while we load your content...
Please wait while we load your content...