Toward enhanced activity of a graphitic carbon nitride-based electrocatalyst in oxygen reduction and hydrogen evolution reactions via atomic sulfur doping†
Abstract
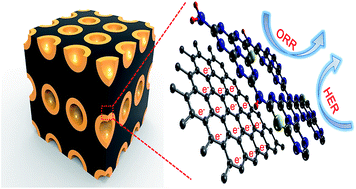
Atomic doping has always been demonstrated as a feasible way to effectively alter the catalytic properties of metal-free electrocatalysts. Herein, we report the first experimental and theoretical investigation regarding the influence of sulfur doping on the activity of a carbon nitride (C3N4)-based electrocatalyst in the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER). It is found that the sulfur dopant within the mesoporous carbon-supported C3N4 motif can remarkably boost its ORR activity, which rivals that of commercial Pt/C yet with better cross-over tolerance and durability, while the HER performance of the composite catalyst is superior than most other reported metal-free electrocatalysts and is even comparable to the most active non-noble metal-based HER materials. Theoretical calculations further reveal that the excellent activity of the doped composite stems from the high charge and spin densities in the C3N4 motif as well as altered competent adsorption energies of reaction intermediates via the atomic sulfur doping. The results in this work feature a facile and effective approach for engineering a high performance C3N4-based electrocatalyst, which may also enlighten the designing and fabrication of other metal-free materials as next-generation electrocatalysts.

 Please wait while we load your content...
Please wait while we load your content...