An ultrasound-assisted wet-chemistry approach towards uniform Mg(BH4)2·6NH3 nanoparticles with improved dehydrogenation properties
Abstract
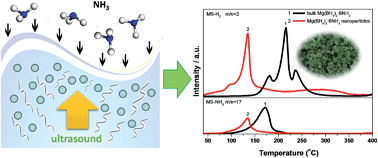
Metal borohydride ammoniates are a novel family of high-capacity hydrogen storage materials. However, high dehydrogenation temperature and low hydrogen purity retard their practical applications. Reducing the particle size to the nanometre range is an effective approach to improve the hydrogen storage properties of hydrides. In this work, we demonstrate a novel ultrasound-assisted wet-chemistry approach to synthesize Mg(BH4)2·6NH3 nanoparticles measuring 20–40 nm in diameter with uniform morphologies. The prepared Mg(BH4)2·6NH3 nanoparticles exhibit dehydrogenation thermodynamics and kinetics much superior to their bulk counterparts because they start releasing hydrogen below 30 °C and peak at 135 °C. More importantly, hydrogen, instead of ammonia, is observed to be the major decomposition product upon heating, thereby representing a substantial advantage. Further investigation revealed that Mg(BH4)2·6NH3 nanoparticles decompose to produce BN and a new Mg–B–N compound instead of Mg. The underlying mechanism of the changed dehydrogenation behaviour of nano-Mg(BH4)2·6NH3 is understood with first-principle calculations.

 Please wait while we load your content...
Please wait while we load your content...