From solid to core@shell to hollow Pt–Ag nanocrystals: thermally controlled surface segregation to enhance catalytic activity and durability†
Abstract
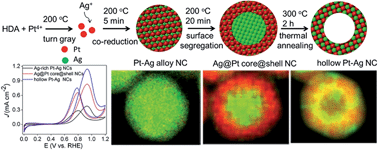
Hollow bimetallic nanostructures exhibit increased durability and utilization efficiency compared to their solid counterparts, and therefore have become promising new candidates for catalytic applications. Here we demonstrate that hollow structured Pt–Ag nanocrystals can be fabricated through a simple one-pot synthesis by employing thermal treatment. During the reaction, Ag-rich Pt–Ag alloy nanocrystals were obtained shortly after co-reduction of Pt and Ag precursors. Subsequent reduction of Pt on the surface of the nanocrystals induced the alloyed Pt atoms to migrate outward to form Ag@Pt core@shell nanocrystals. They finally evolved into hollow alloy structures at an elevated temperature due to the surface energy difference of the metals and the Kirkendall effect. The hollow Pt–Ag nanocatalysts exhibited a substantial enhancement in the current density at 0.9 V and CO-tolerance toward the methanol oxidation reaction, and also showed excellent durability in acid media for methanol oxidation and in alkaline media for p-nitrophenol reduction. This new method of synthesizing hollow nanocrystals can be used towards the design and fabrication of a wide range of Pt-based bimetallic hollow nanostructures.
- This article is part of the themed collection: 2016 Journal of Materials Chemistry A HOT Papers

 Please wait while we load your content...
Please wait while we load your content...