Manganous oxide nanoparticles encapsulated in few-layer carbon as an efficient electrocatalyst for oxygen reduction in alkaline media†
Abstract
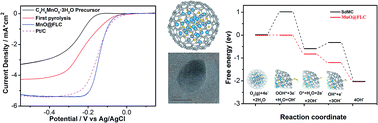
To replace Pt-based catalysts for the oxygen reduction reaction (ORR) in energy conversion and storage systems, it is highly desirable to explore low-cost and high-performance nonprecious metal catalysts. Herein, we report a novel Mn-based catalytic system for the ORR which consists of MnO nanoparticles encapsulated in mesoporous few-layer carbon (MnO@FLC). Using density functional theory (DFT), we disclose that the few-layer carbon could be activated by the encapsulated MnO nanoparticles and further become active sites for the ORR process. The MnO@FLC electrocatalyst exhibits outstanding ORR activity (an onset potential of −0.005 V vs. Ag/AgCl, a half-wave potential of −0.153 V and a limiting current density of −5.38 mA cm−2), which are among the best performances reported to date for Mn-based ORR electrocatalysts and highly comparable to those of state-of-the-art Pt/C catalysts in alkaline media. Our work suggests that metal oxides encapsulated in few-layer carbon could serve as efficient ORR electrocatalysts with decent performance.

 Please wait while we load your content...
Please wait while we load your content...