Microporous LiAlSiO4 with high ionic conductivity working as a coating material and water adsorbent for LiNi0.5Mn1.5O4 cathode†
Abstract
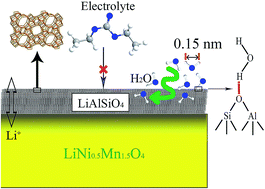
Trace water in the electrolyte can cause serious capacity fading, especially for the high voltage LiNi0.5Mn1.5O4 (LNM) cathode. Here, we coat microporous LiAlSiO4 on LNM particles using a citric acid-based sol–gel method. LiAlSiO4 has been reported to be a good one-dimensional Li-ion conductor, ensuring that Li+ ions could cross this coating layer easily. Simultaneously, this LiAlSiO4 coating layer contains many micropores with a radius of less than 2 nm, which could anchor H2O molecules. The adsorption of H2O suppresses the formation of HF, which further suppresses Mn dissolution of the LNM material, especially at high temperature. Finally, the LiAlSiO4-modified LNM displays an obviously improved cycling stability, maintaining a capacity retention of 94.36% after 150 cycles at 1C and 55 °C.

 Please wait while we load your content...
Please wait while we load your content...