A hollow spherical doped carbon catalyst derived from zeolitic imidazolate framework nanocrystals impregnated/covered with iron phthalocyanines†
Abstract
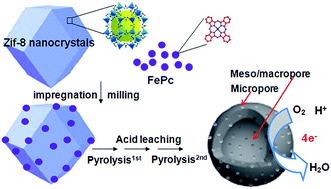
A hollow spherical doped carbon catalyst with a large surface area and hierarchical porous structure is prepared by pyrolyzing zeolitic imidazolate framework nanocrystals (Z8Ncs) impregnated/covered with iron phthalocyanines (FePcs). It is found that the doping of FePcs into the Z8Nc precursor plays a crucial role in the structural evolution of the resulting hollow-core porous carbon as well as its high catalytic performance. Doped carbon catalysts derived from either Z8Ncs or FePcs exhibit poor activity towards oxygen reduction, whereas the catalyst derived from Z8Ncs impregnated/covered with FePcs exhibits extremely high performance in both acidic and alkaline media. In 0.1 M HClO4, its onset potential reaches up to 0.910 V, and its half-potential (0.790 V) is only 60 mV lower than that of the 20 wt% Pt/C catalyst (0.850 V). In 0.1 M KOH, its ORR activity even surpasses that of Pt/C. We suggest that the high performance of the catalyst is attributable to the following factors: (i) the high active site density caused by doping FePcs into/onto the highly porous, N-rich Z8Ncs, (ii) the high surface area and adequate active site exposure caused by its hollow spherical morphology, and (iii) the hierarchical porous structure which further facilitates the diffusion and adsorption of oxygen molecules.
- This article is part of the themed collection: 2016 Journal of Materials Chemistry A HOT Papers

 Please wait while we load your content...
Please wait while we load your content...