pH-regulative synthesis of Na3(VPO4)2F3 nanoflowers and their improved Na cycling stability†
Abstract
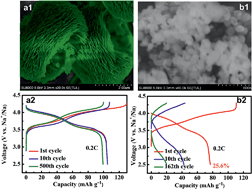
Na-ion batteries are becoming increasingly attractive as a low cost energy storage device. Sodium vanadium fluorophosphates have been studied extensively recently due to their high storage capacity and high discharge voltage. Shape and size often have a crucial influence over the properties. The controlling synthesis of nanoparticles with special microstructures is significant, which becomes a challenging issue and has drawn considerable attention. In this study, Na3(VPO4)2F3 nanoflowers have been synthesized via a pH-regulative low-temperature (120 °C) hydro-thermal route. In particular, it is a green route without any organic compounds involved. The hydro-thermal reaction time for the formation of Na3(VPO4)2F3 nanoflowers has also been investigated. A weak acid environment (pH = 2.60) with the possible presence of hydrogen fluoride molecules is necessary for the formation of the desired nanoflower microstructures. Compared to the nanoparticles obtained by Na2HPO4·12H2O, the as-synthesized Na3(VPO4)2F3 nanoflowers showed an excellent Na-storage performance in terms of superior cycle stability, even without any further carbon coating or high-temperature treatment.

 Please wait while we load your content...
Please wait while we load your content...