Influence of particle size, cycling rate and temperature on the lithiation process of anatase TiO2†
Abstract
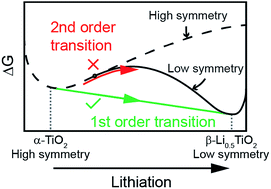
The nature of a phase transition plays an important role in controlling the kinetics of reaction of an electrode material in a lithium-ion battery. The actual phase transition path can be affected by particle size and cycling rate. In this study, we investigated the phase transition process during the electrochemical Li intercalation of anatase TiO2 as a function of particle size (25 nm and 100 nm), cycling rate (1C, 2C, 5C, 10C, 20C) and temperature (room temperature and 80 °C) by in situ synchrotron X-ray diffraction. The phase transition was found to be affected by the particle size: the 100 nm particles react simultaneously via a conventional nucleation and growth, i.e. two-phase, mechanism, while the 25 nm particles react sequentially via a two-phase mechanism. The Li miscibility gap decreases with increasing cycling rate, yet the phase separation was not suppressed even at a cycle rate of 20C. An increase in temperature from room temperature to 80 °C significantly improves the electrode's electrochemical performance despite undergoing a two-phase reaction. The failure to observe a continuous structural transition from tetragonal TiO2 to orthorhombic Li0.5TiO2 even at high rates and elevated temperature was attributed to the high energy barrier of a continuous phase transition path.

 Please wait while we load your content...
Please wait while we load your content...