Seaweed biomass derived (Ni,Co)/CNT nanoaerogels: efficient bifunctional electrocatalysts for oxygen evolution and reduction reactions†
Abstract
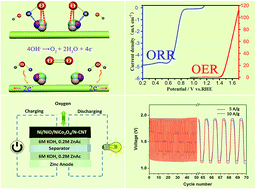
Developing earth-abundant, active and stable electrocatalysts which operate in two-electrode rechargeable metal–air batteries, including both oxygen evolution and reduction reactions (OER and ORR), is vital for renewable energy conversion in real application. Here, we demonstrate a three-dimensional (3D) bifunctional nanoaerogel electrocatalyst that exhibits good electrocatalytic properties for both OER and ORR. This material was fabricated using a scalable and facile method involving the pyrolysis of (Ni,Co)/CNT alginate hydrogels derived from sustainable seaweed biomass after an ion exchange process. The bifunctionality for oxygen electrocatalysis as shown by the OER–ORR potential difference (ΔE, the OER and ORR potentials are taken at the current densities of 10 mA cm−2 and −3 mA cm−2 in 0.1 M KOH, respectively) could be reduced to as low as 0.87 V, comparable to the state-of-the-art non-noble bifunctional catalysts. The good performance was attributed to the ternary Ni/NiO/NiCo2O4 catalytic center for charge transfer and 3D hierarchical mesoporous hybrid framework for efficient mass transport. More importantly, the Zn–air battery fabricated with the hybrid nanoaerogel as a bifunctional electrocatalyst displays very high energy efficiency (58.5%) and long-term stability. Prospectively, our present work may pave a new way to develop earth-abundant and low cost high-performance bifunctional electrocatalysts for rechargeable metal–air batteries.

 Please wait while we load your content...
Please wait while we load your content...