Highly stable poly(ethylene glycol)-grafted alkaline anion exchange membranes†
Abstract
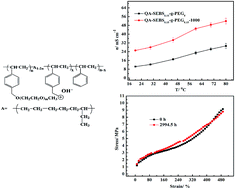
A mechanically and chemically stable poly(ethylene glycol) (PEG)-grafted poly(styrene-ethylene-co-butylene-styrene) (SEBS)-based alkaline anion exchange membrane (AAEM) was designed, prepared and characterized. When subjected to tensile strain, the elongation at breaking of these SEBS-based AAEMs was up to 500%, a value 80 times greater than that of an AAEM using polystyrene as the main chain. Remarkably, the ion exchange capacity (IEC), conductivity, dimensions and mechanical properties of this AAEM could remain almost unchanged in 2.5 M KOH at 60 °C for about 3000 h, indicating the excellent alkaline stability of the PEG-grafted SEBS-based AAEMs. As confirmed by TEM, the grafting of PEG could enlarge the size of the ion-conducting channels, significantly enhancing the conductivity of these AAEMs (80 °C, from 29.2 mS cm−1 to 51.9 mS cm−1). Furthermore, the peak power density of an H2/O2 single fuel cell using this SEBS-based AAEM was up to 146 mW cm−2 at 50 °C. Based on these outstanding properties, this membrane has potential application not only for fuel cells, but also for other electrochemical energy conversion and storage devices, such as redox flow and alkaline ion batteries.

 Please wait while we load your content...
Please wait while we load your content...