Ammonium tetrathiomolybdate as a novel electrode material for convenient tuning of the kinetics of electrochemical O2 reduction by using iron–porphyrin catalysts†
Abstract
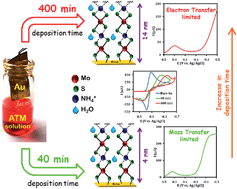
Ammonium tetrathiomolybdate (ATM) is found to be a novel electrode material forming self-assembled adlayers on Au electrodes which are robust and stable in aqueous environments. Iron–porphyrin catalysts can be physiadsorbed on this electrode material and these modified electrodes are quite stable during hydrodynamic electrochemical experiments unlike self-assembled monolayers (SAMs) of thiols on Au/Ag. The rate of interfacial charge transfer can be tuned by controlling the height of the ATM assembly by varying the deposition time. These electrodes allow convenient tuning of the electrokinetics of 4e−/4H+ O2 reduction from mass transfer to charge transfer with the same electrode material.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials

 Please wait while we load your content...
Please wait while we load your content...