In situ surface alkalinized g-C3N4 toward enhancement of photocatalytic H2 evolution under visible-light irradiation†
Abstract
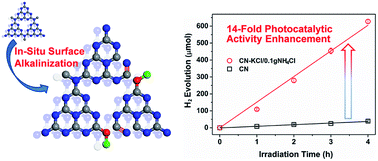
Surface-alkalinization over g-C3N4 was realized by an in situ synthesis approach of introducing KCl and NH4Cl during the polymerization of melamine. The characterization of the Fourier transform-infrared spectrum, X-ray photoelectron spectrum, and electron spin resonance spectrum over the sample synthesized in the presence of KCl/NH4Cl and other reference samples indicated that the K ions played an essential role in breaking the periodic chemical structure of g-C3N4 and meanwhile the trace amount of H2O in melamine could supply OH ions to graft hydroxyl groups. The NH4Cl mainly contributed to exfoliation of layered g-C3N4 particles and pushing negative shift of the conduction-band level based on the measurements of the BET surface area and valence-band X-ray photoelectron spectrum. An optimal sample, g–C3N4–KCl/0.1 g NH4Cl (CN–KCl/0.1 g NH4Cl), achieved a more than 14-fold enhancement in photocatalytic H2 evolution under visible-light irradiation compared with the pristine g-C3N4. The enhanced photocatalytic efficiency could be attributed to the fact that the surface hydroxyl groups and the more negative conduction-band level can promote the separation of photocarriers and offer a stronger potential for water reduction, respectively.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...