Workfunction, a new viewpoint to understand the electrolyte/electrode interface reaction†
Abstract
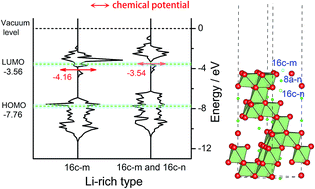
Severe gassing during cycling hinders the application of spinel Li4Ti5O12 as a zero-strain anode material for constructing high power-density and long-lifespan Li-ion batteries. The gassing issue is caused by the interface reaction between Li4Ti5O12 and the electrolyte. This article is aimed towards understanding the Li4Ti5O12/electrolyte interface reaction and the chemical stability of Li4Ti5O12 from a new viewpoint, surface workfunction, the energy required to take away one electron from the Fermi level. Density functional theory (DFT) calculations indicate that the workfunction decreases due to the presence of the Li-rich surface (Li+-occupied 16c sites) of Li3+xTi6−xO12. Meanwhile, the chemical potential increases and even reaches the LUMO of the carbonate electrolyte, easily inducing interface reactions. This means that the electrochemically lithiated phase Li7Ti5O12 is responsible for the electrolyte decomposition on the surface. The Li-rich surfaces of Li4Ti5O12 generated during material preparation or chemical lithiation can also trigger the interface reactions. From the combination of the experimental and calculation results, we believe that the interface reaction involves losses of electrons and Li+ ions from the Li-rich surface of Li4Ti5O12 or Li7Ti5O12, and the reduction of the electrolyte. In addition, O vacancies on the surface decrease the workfunction and further promote the reaction.

 Please wait while we load your content...
Please wait while we load your content...