Photo-assisted water splitting with bipolar membrane induced pH gradients for practical solar fuel devices†
Abstract
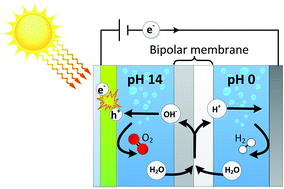
Different pH requirements for a cathode and an anode result in a non-optimal performance for practical solar fuel systems. We present for the first time a photo-assisted water splitting device using a bipolar membrane, which allows a cathode to operate in an acidic electrolyte while the photoanode is in alkaline conditions. The bipolar membrane dissociates water into H+ and OH−, which is consumed for hydrogen evolution at the cathode and oxygen evolution at the anode, respectively. The introduction of such a bipolar membrane for solar fuel systems provides ultimate freedom for combining different (photo)cathodes and -anodes. This paper shows that photo-assisted water splitting at both extreme pH gradients (0–14) as well as mild pH gradients (0–7) yields current densities of 2–2.5 mA cm−2 using a BiVO4 photoanode and a bipolar membrane. The membrane potentials are within 30 mV of the theoretical electrochemical potential for low current densities. The pH gradient is maintained for 4 days of continuous operation and electrolyte analysis shows that salt cross-over is minimal. The stable operation of the bipolar membrane in extreme and mild pH gradients, at negligible loss, contributes to a sustainable and practically feasible solar fuel device with existing photoactive electrodes operating at different pH.

 Please wait while we load your content...
Please wait while we load your content...