Hypercross-linked microporous polymeric ionic liquid membranes: synthesis, properties and their application in H2 generation
Abstract
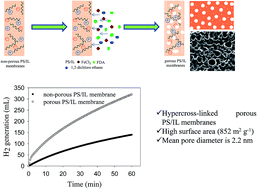
This work focuses on hydrogen (H2) generation from the hydrolysis of sodium borohydride (NaBH4) by using high performance microporous polymeric ionic liquid membranes. Microporous organic polymers are broadly recognized for gas separation and gas storage. In this study, we synthesized hypercross-linked microporous polystyrene (PS) ionic liquid (IL) membranes via in situ cross-linking by the Friedel–Crafts alkylation method. The BET surface area of microporous PS/IL membranes after cross-linking is as high as 852 m2 g−1 compared to non-porous PS/IL membranes before cross-linking (2.7 m2 g−1) and pure PS membranes (2.5 m2 g−1). The as-prepared microporous membranes are tested for H2 generation. The results show that the microporous PS/IL membranes increase the H2 rate as compared to non-porous membranes. This is due to the dominant factor of hierarchical porosity and high surface area of the membranes. There is no leach or loss of the ionic liquid; it is well attached to the cross-linked membranes. The highest rate of H2 generation (3621 mL H2 g−1 min−1) was observed for 8 wt% of NaBH4 solution, which is comparable to that of noble metal-based catalysts reported in the literature for a similar kind of this reaction. The catalyst provided 17 290 total turnovers before they are deactivated.

 Please wait while we load your content...
Please wait while we load your content...