VO2F: a new transition metal oxyfluoride with high specific capacity for Li ion batteries†
Abstract
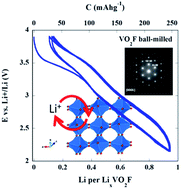
Hitherto unreported vanadium oxyfluoride VO2F has been synthesized using a solid state reaction at a pressure of 4 GPa and 800 °C. This long awaited vanadium oxyfluoride fills the existing gap of ReO3-type MO2F compounds of Group 5 elements, from which only NbO2F and TaO2F have been known to exist to date. VO2F crystallizes with the VF3-type structure, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, with a = 5.1226(1) Å and c = 13.0686(3) Å as determined by powder X-ray diffraction. Highly structured diffuse streaking observed in electron diffraction patterns evidences local O/F ordering. VO2F exhibits two regions upon discharge in a lithium cell, an upper sloped region in the range of 3.9–2.2 V and a lower plateau at 2.15 V. Discharge of VO2F to 1 V provides a gravimetric capacity of 450 mA h g−1. VO2F can reversibly insert up to 1 Li+ per vanadium above 2.15 V without destruction of the host structure, delivering a gravimetric capacity as high as 250 mA h g−1 and pointing to VO2F as a promising intercalation electrode.
c, with a = 5.1226(1) Å and c = 13.0686(3) Å as determined by powder X-ray diffraction. Highly structured diffuse streaking observed in electron diffraction patterns evidences local O/F ordering. VO2F exhibits two regions upon discharge in a lithium cell, an upper sloped region in the range of 3.9–2.2 V and a lower plateau at 2.15 V. Discharge of VO2F to 1 V provides a gravimetric capacity of 450 mA h g−1. VO2F can reversibly insert up to 1 Li+ per vanadium above 2.15 V without destruction of the host structure, delivering a gravimetric capacity as high as 250 mA h g−1 and pointing to VO2F as a promising intercalation electrode.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...