Hydration, oxidation, and reduction of GdBaCo2O5.5 from first-principles
Abstract
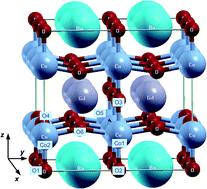
Structural, magnetic and chemical properties of GdBaCo2O5.5 regarding hydration, oxidation and reduction are studied by density-functional calculations in the DFT + U formalism. Besides the orthorhombic Pmmm structure, a lower energy, tilted, distorted structure is found. The hydrated configurations obtained by water dissociation in the oxygen vacancies of the GdO0.5 plane exhibit strong distortions with respect to the Pmmm structure. Simulation of protonic defects provides the energy landscape of incorporated protons, which preferentially bind to oxygens of the CoO2 planes, suggesting their possible bidimensional diffusion in this plane. We also studied oxygen incorporation (oxidation) in the oxygen vacancies of the GdO0.5 planes, and oxygen removal (reduction) from BaO, CoO2 and GdO0.5 planes. The oxidized compound, GdBaCo2O5.75, is rather p-type metallic, while the reduced compound, GdBaCo2O5.25, remains an insulator, due to electronic localization (Co3+ + e− → Co2+). Taking Pmmm as the reference, both hydration at high water concentration (one H2O per 38-atom supercell) and oxidation are found exothermic. However, if the distorted structure is chosen as the reference, these reactions become endothermic, at least at the high water/oxygen concentration studied. Reduction is, in both cases, endothermic. Nevertheless, negative formation energies of the protonic defects suggest the possibility of hydration at lower water concentration.

 Please wait while we load your content...
Please wait while we load your content...