Adsorption characteristics of noble metal ions onto modified straw bearing amine and thiol groups
Abstract
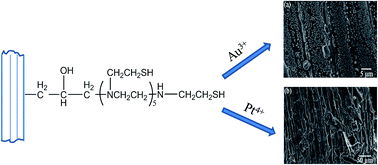
Wheat straw was chemically modified by introducing amine and thiol groups in order to improve its adsorption capacity and selective adsorption ability towards noble metal ions. The removal of Au3+ and Pt4+ ions from aqueous solutions was assessed by using a batch sorption technique. The maximum adsorption amount for Au3+ and Pt4+ ions reached as high as 450 and 380 mg g−1, and the adsorption equilibrium could be achieved within 120 min. The adsorption mechanism studied by X-ray photoelectron spectroscopy (XPS) revealed that the sorption of noble metal ions was realized by ion-exchange, coordination and redox reactions. The selectivity studies suggested that the quantitative removal and determination of Au3+ and Pt4+ ions could be achieved even in the presence of an excess of interfering ions. The desorption efficiency of adsorbed precious metals was studied by using various concentrations of HCl, NaOH, thiourea and thiourea–HCl solutions. It was indicated that the 0.8 mol L−1 thiourea–0.5 mol L−1 HCl solution could effectively desorb Au3+ and Pt4+ ions from the modified straw.

 Please wait while we load your content...
Please wait while we load your content...