Draining the photoinduced electrons away from an anode: the preparation of Ag/Ag3PO4 composite nanoplate photoanodes for highly efficient water splitting†
Abstract
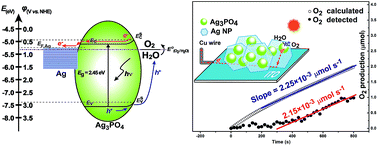
Ag/Ag3PO4 composite nanoplate photoanodes were fabricated by electrogeneration of Ag3PO4 on the surface of vertically aligned Ag nanoplates (NPs) in phosphate solution. The outside Ag3PO4 layer acted as a light-absorbing material to generate electron–hole pairs, while the inside Ag NPs acted as both the framework and the electrical connector between Ag3PO4 and the conducting substrate. The obtained composite photoanodes showed a high catalytic activity toward photoelectrochemical (PEC) oxygen evolution reaction (OER). The photoinduced holes reacted with water to generate oxygen on the Ag3PO4 surface, while the photoinduced electrons were efficiently transported to the counter electrode by highly conductive Ag NPs. The Ag/Ag3PO4 composite photoanode exhibited a photocurrent density of 0.25 mA cm−2 at 0.500 V vs. SCE, which is the highest among reported values obtained under conditions similar to this work. The amount of evolved oxygen was monitored to evaluate the percentage of the photocurrent involved in PEC OER, and the Faraday efficiency for PEC OER was obtained to be ca. 95.6%, indicating that most of the photoinduced holes were engaged in OER. The in situ PEC oxidation of Ag to Ag3PO4, which compensated the loss of Ag3PO4 during PEC OER, makes the Ag/Ag3PO4 composite a self-healing system for OER in phosphate solution.

 Please wait while we load your content...
Please wait while we load your content...