A novel solid-state electrolyte based on a crown ether lithium salt complex†
Abstract
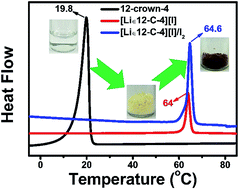
A novel crown ether lithium salt complex [Li∈12-C-4][I] has been designed, synthesized and characterized. The thermal properties of the [Li∈12-C-4][I] based solid-state electrolytes were also investigated in detail. The particular trapping ability of 12-crown-4 to Li+ can obviously reduce the cation–anion (Li+–I−) interaction and hence facilitate favorable electrical properties of the solid-state electrolytes. Therefore, [Li∈12-C-4][I] represents ionic conductivity of 3.93 × 10−5 and 1.53 × 10−4 S cm−1 at 25 and 80 °C, respectively. Further addition of the ionic liquid 1-propyl-3-methylimidazolium iodine as a crystal growth inhibitor can effectively suppress the crystallization of the complex for more amorphous and smoother regions, which are much more conducive towards higher ion conductivity by the segmental motion of molecule chains. For application, the resulting device showed a power conversion efficiency of 5% and displayed excellent long-term stability. These results offer us more opportunities to explore simple and novel solid-state electrolytes for energy storage and conversion.

 Please wait while we load your content...
Please wait while we load your content...