Binary transition metal nitrides with enhanced activity and durability for the oxygen reduction reaction†
Abstract
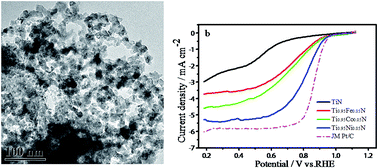
With a novel two-step approach, we prepared a low-cost, high-performance, binary transition metal nitride (BTMN) catalyst. An ammonia (NH3) complex of Ti and a transition metal was prepared in an organic solvent by the reaction of metal ions with ammonium; the complex then was dried in a vacuum oven, followed by nitridation in a tubular furnace under NH3 flow. The catalyst exhibited excellent activity towards the oxygen reduction reaction (ORR) in an alkaline medium and good ORR activity in an acidic medium. The effects of the doping elements (Fe, Co, and Ni), the doping concentration, and various nitriding temperatures on catalytic performance were intensively investigated. The onset potential of the Ti0.95Ni0.5N catalyst reached 0.83 V, with a limiting diffusion current density of 4 mA cm−2 (at a rotation speed of 1600 rpm) in 0.1 M HClO4 solution, which is the highest to date among the reported TiN-based electrocatalysts in an acidic medium. In 0.1 M KOH solution, the performance of this catalyst was almost comparable to that of commercial JM Pt/C; the diffusion current density reached 5.3 mA cm−2, and the halfway potential was only 71 mV inferior to that of commercial JM Pt/C. Furthermore, the catalyst showed high stability and only a slight drop in its current density after the durability test. All of these findings make our BTMN catalyst attractive for PEMFCs.

 Please wait while we load your content...
Please wait while we load your content...