β-Cyclodextrin stabilized magnetic Fe3S4 nanoparticles for efficient removal of Pb(ii)†
Abstract
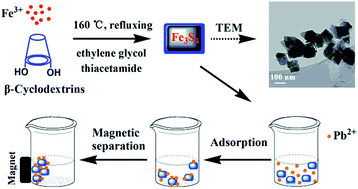
In this study, magnetic β-cyclodextrin stabilized Fe3S4 nanoparticles (CD-Fe3S4) were fabricated via a facile one-step route. The as-prepared CD-Fe3S4 exhibited a high Pb(II) adsorption capacity of 256 mg g−1, and it also showed a surprisingly efficient adsorption toward Zn(II), Cd(II), and Cu(II). XRD, FTIR and XPS analyses suggested that the mechanisms governing Pb(II) removal by CD-Fe3S4 were precipitation (formation of galena) and surface adsorption. Additionally, the magnetic properties of the synthesized Fe3S4 nanoparticles allow fast separation of sorbents from water. The sorption kinetic data were well-described by a pseudo-second-order model, whereas the adsorption isotherms followed a Langmuir isotherm model. Our experimental results proved that the β-cyclodextrin stabilized Fe3S4 could be a promising candidate for the removal of Pb(II) from water via simultaneously taking advantage of its magnetic properties and high affinity for heavy metals.

 Please wait while we load your content...
Please wait while we load your content...